
When you're developing an OTC brand, choosing the right packaging method can make or break your profitability. The decision between unit dose packaging and traditional packaging formats affects everything from manufacturing costs to patient compliance rates. As the pharmaceutical packaging industry evolves, understanding these differences is crucial for your business success.
The reality is that unit dose packaging delivers superior economics and patient outcomes for most OTC brands, despite higher material costs. While traditional packaging methods remain viable for specific scenarios, the data overwhelmingly favors unit dose systems for growing brands focused on quality, compliance, and long-term profitability.
Unit Dose Packaging: The Modern Manufacturing Advantage
Unit dose packaging represents the most advanced approach to OTC pharmaceutical packaging available today. This method pre-packages exact doses in individual containers, eliminating guesswork and reducing contamination risks throughout the supply chain.
Cost Efficiency Despite Higher Materials
The most compelling advantage of unit dose packaging is its paradoxical cost structure. While material costs increase by 15-25% compared to traditional methods, total manufacturing costs decrease by an impressive 29%. This translates to net savings of 12-28% on your bottom line. The secret lies in operational automation that eliminates labor-intensive processes and reduces waste streams.
Labor Cost Revolution
Automated unit dose systems reduce labor costs by 40-60% compared to traditional bulk operations. Your production team no longer needs to manually measure, count, or verify individual doses. Instead, sophisticated machinery handles these tasks with 100% consistency and accuracy. This labor efficiency becomes particularly valuable as your production volumes scale.
Unmatched Quality Control Standards
Every unit dose package undergoes 100% automated verification without human intervention. This level of quality assurance has produced an 89% reduction in contamination-related batch failures in real-world manufacturing environments. For OTC brands where product safety directly impacts your reputation, this quality advantage is invaluable.
Enhanced Patient Adherence
Pre-measured doses eliminate calculation errors that plague traditional packaging formats. Each package includes printed dosing instructions that act as built-in reminders for patients. This feature is especially valuable for OTC products targeting seniors or patients managing complex medication regimens.
Traditional Packaging: Established But Limited
Traditional packaging methods: primarily bottles and blister packs: represent proven systems that have served the pharmaceutical industry for decades. These approaches offer certain advantages for specific business scenarios.
Lower Capital Investment Requirements
Traditional packaging requires significantly less upfront equipment investment. Small-scale operations can begin production with minimal capital outlay, making this approach attractive for startups or businesses testing new product concepts.
Established Supply Chains
Most packaging suppliers and contract filling companies already possess infrastructure optimized for traditional formats. This established ecosystem means faster setup times and readily available technical support for standard operations.
Manual Process Flexibility
For extremely small batch runs, manual traditional packaging processes offer immediate flexibility without equipment setup requirements. However, this advantage disappears as production volumes increase beyond artisanal levels.
Head-to-Head Performance Comparison
| Performance Metric | Unit Dose Packaging | Traditional Packaging |
|---|---|---|
| Total Manufacturing Cost | $0.25 per unit (29% reduction) | $0.35 per unit |
| Labor Efficiency | 40-60% cost reduction through automation | Manual processes; higher labor intensity |
| Waste Reduction | 75% waste reduction ($0.01 vs $0.04 per unit) | Higher material waste and rework |
| Regulatory Compliance | 60% compliance cost reduction | Traditional documentation burden |
| Quality Control | 100% automated verification | Statistical sampling with manual steps |
| Batch Flexibility | 10,000 to 500,000+ units efficiently | Requires larger minimums for cost-effectiveness |
| Production Changeover | 30-60 minutes between products | 2-4 hours changeover time |
| Contamination Risk | Individual sealing minimizes cross-contamination | Higher contamination potential |
| Market Positioning | Enables 35% price premium | Limited premium positioning ability |
Financial Impact: The Three-Year Reality
Real-world case studies reveal the true financial impact of packaging choice decisions. Unit dose operations generate approximately $1,340,000 annual profit compared to $847,000 for traditional bulk packaging: representing an additional $493,000 annually (58% higher profit) for the same product volume.
This profit advantage stems from multiple sources: reduced operational costs, premium pricing capability, lower regulatory compliance expenses, and dramatically reduced waste streams. The 35% price premium enabled by unit dose positioning substantially enhances your profitability timeline.
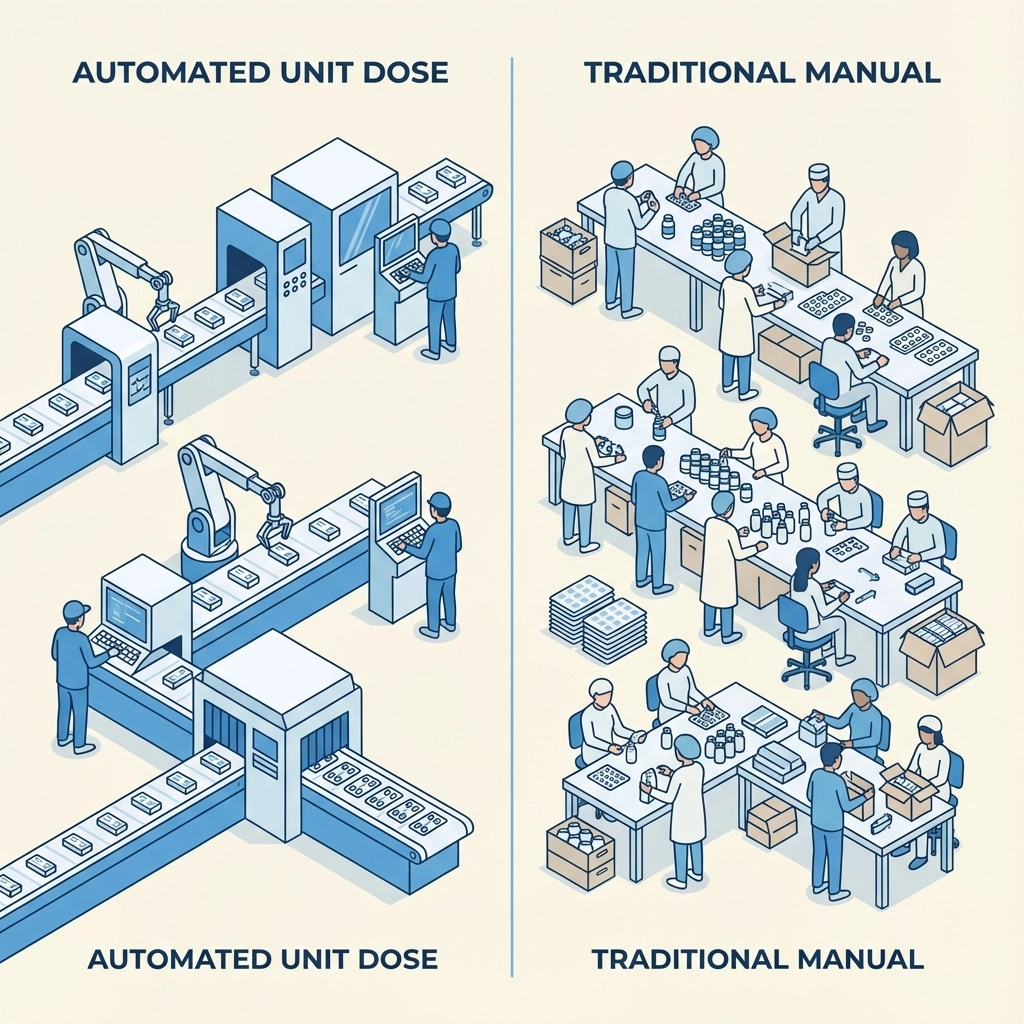
Making the Right Choice for Your OTC Brand
Choose Unit Dose Packaging When:
Your target market values convenience and safety: particularly seniors, patients with complex regimens, or health-conscious consumers seeking premium products. Unit dose packaging excels when you plan production runs of 10,000+ units and your product supports premium market positioning.
This approach is ideal when regulatory compliance and track-and-trace requirements are critical to your business model. If long-term profitability matters more than initial setup costs, and you require production flexibility across multiple SKUs, unit dose packaging delivers superior results.
Choose Traditional Packaging When:
You operate at extremely small production volumes under 10,000 units, or budget constraints require minimal capital investment. Traditional packaging works best when your product commands no significant price premium and distribution channels specifically prefer traditional formats.
Consider traditional methods if you lack access to experienced turnkey manufacturing partnerships with unit dose capabilities, or when your product characteristics don't align with automated packaging processes.
The Partnership Advantage
Success with unit dose packaging requires partnering with experienced contract packaging specialists who understand both the technical requirements and market dynamics of OTC pharmaceutical packaging. The most effective partnerships combine clean room manufacturing standards with comprehensive regulatory expertise.
Leading contract packagers offer complete turnkey operations that handle everything from initial product assessment through final packaging and distribution. This comprehensive approach eliminates the complexity of coordinating multiple vendors while ensuring consistent quality standards throughout your supply chain.
Frequently Asked Questions
Q: How much does unit dose packaging cost compared to traditional methods?
A: While material costs are 15-25% higher, total manufacturing costs decrease by 29%, resulting in net savings of 12-28%. The break-even point typically occurs within the first production year.
Q: What minimum production volumes make unit dose packaging cost-effective?
A: Unit dose systems handle batch sizes from 10,000 to 500,000+ units efficiently. Below 10,000 units, traditional packaging may be more cost-effective due to setup overhead.
Q: Does unit dose packaging work for all OTC product types?
A: Unit dose packaging accommodates liquids, powders, capsules, tablets, and topical formulations. Product-specific assessments determine optimal packaging configurations for each formulation.
Q: How long does it take to transition from traditional to unit dose packaging?
A: Timeline depends on product complexity and regulatory requirements. Typical transitions take 3-6 months from initial assessment to full production, including equipment validation and regulatory approval processes.
Q: What regulatory advantages does unit dose packaging provide?
A: Unit dose systems simplify GMP compliance through automated documentation, individual package traceability, and reduced contamination risks. This translates to 60% lower regulatory compliance costs.
Ready to Transform Your OTC Brand?
The choice between unit dose packaging and traditional packaging ultimately determines your brand's competitive position in the evolving OTC marketplace. With superior economics, enhanced patient compliance, and premium positioning capability, unit dose packaging represents the future of pharmaceutical packaging.
LF of America's turnkey packaging solutions eliminate the complexity of transitioning to unit dose systems while ensuring optimal results for your specific product requirements. Our experienced team handles everything from initial product assessment through final packaging and distribution, delivering the expertise you need to maximize your packaging investment.
Contact us today to discover how unit dose packaging can transform your OTC brand's profitability and market position. Visit our YouTube channel for detailed case studies, or connect with our team through Facebook for the latest industry insights and packaging innovations.


